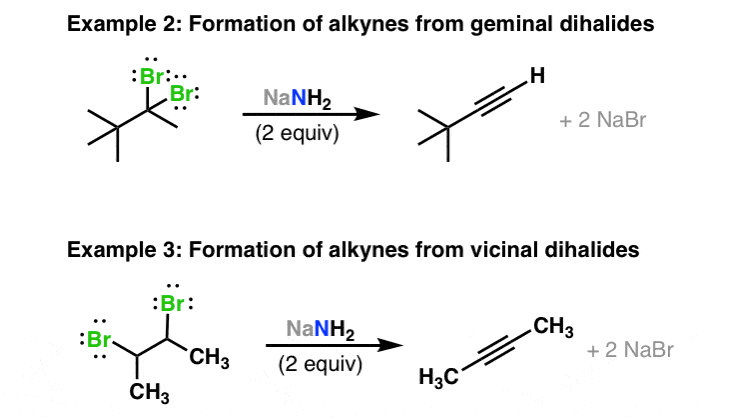
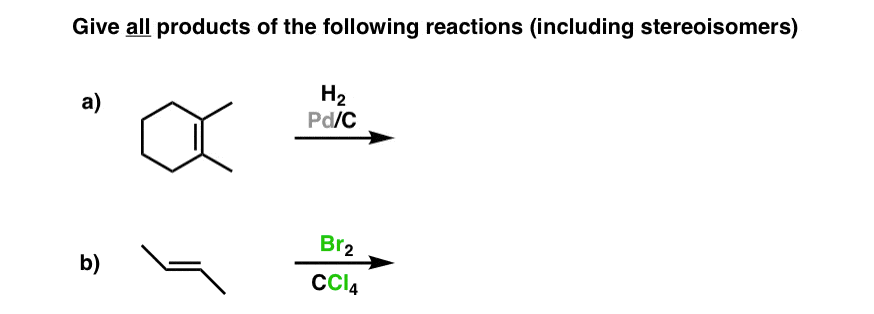
Where Are Vinyl Hydrogens Nmr

Proton nuclear magnetic resonance proton nmr hydrogen 1 nmr or 1 h nmr is the application of nuclear magnetic resonance in nmr spectroscopy with respect to hydrogen 1 nuclei within the molecules of a substance in order to determine the structure of its molecules.
Where are vinyl hydrogens nmr. Key difference allyl vs vinyl both allyl and vinyl groups have slightly similar structures with a small variation. Spectra pdf form of more than 600 compounds are also. Vinyl aromatics nitriles rocr 3 arcr 2 h alkyne r 3coh o 1h 2nmr shift ranges δ ppm vinyl r 3c f r 3c clrc i r 3c br rccr 3 o δ ppm 13c nmr shift ranges r 2nh r 2ncr h approximate nmr shift ranges note. This set of pages originates from professor hans reich uw madison structure determination using spectroscopic methods course chem 605.
These are typical chemical shifts. The key difference between geminal and vicinal coupling is that geminal coupling refers to the coupling of two hydrogen atoms that are bound to the same carbon atom. Tertiary hydrogen vinyl group vinyl chloride vinylic carbocation. It describes nuclear magnetic resonance nmr in details relevant to organic chemistry.
In all of the examples of spin spin coupling that we have seen so far the observed splitting has resulted from the coupling of one set of hydrogens to just one neighboring set of hydrogens. Substituents can move the resonance out of the listed range esters amides acids ketones aldehydes. In samples where natural hydrogen h is used practically all the hydrogen consists of the isotope 1 h hydrogen 1. The greater the substitution on the carbon bearing the hydrogen the further downfield higher frequency the resonance occurs.
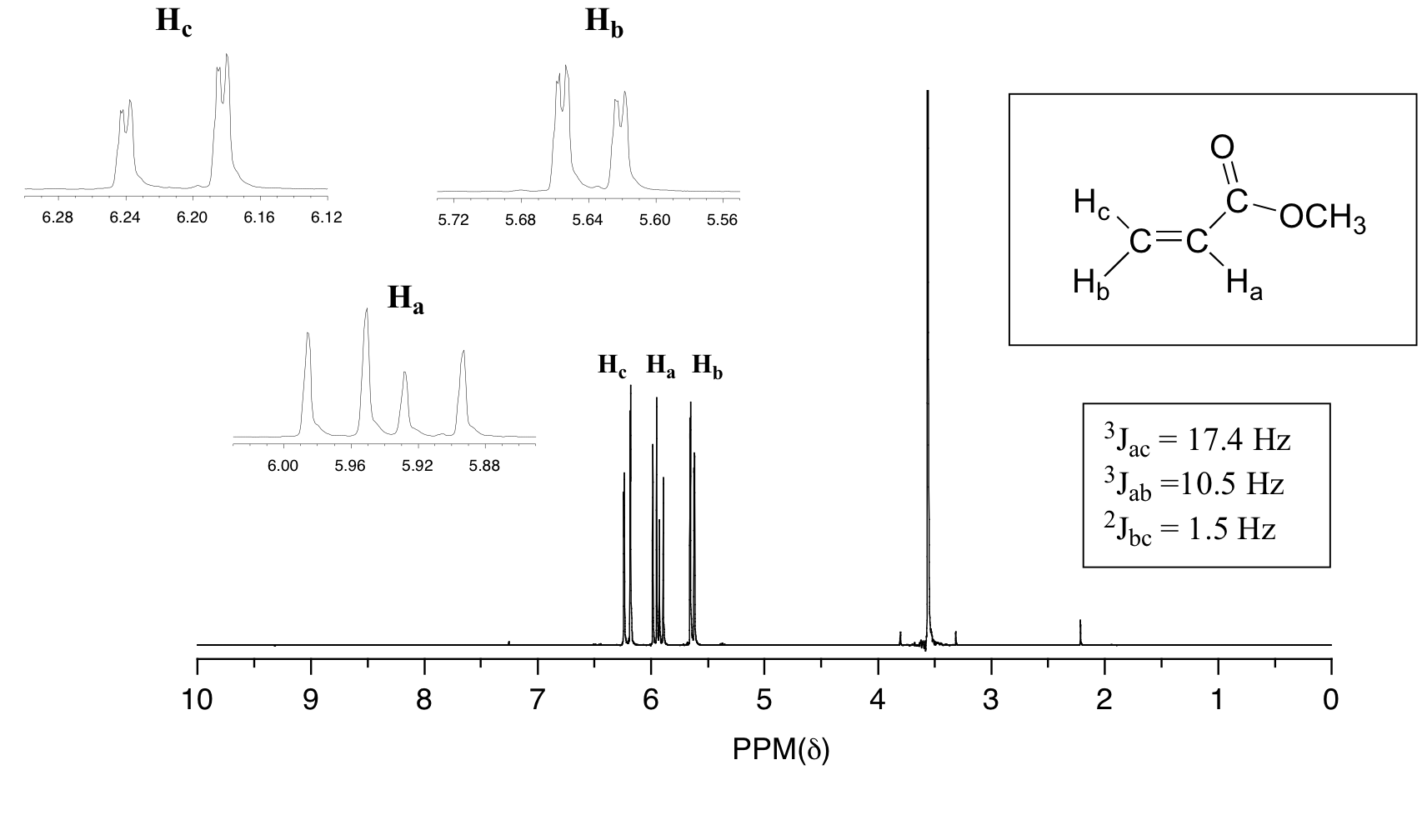
Allyl groups have three carbon atoms and five hydrogen atoms. None of the other hydrogens are vinylic. The vinylic hydrogens are shown in red. For vinylic hydrogens in a trans configuration we see coupling constants in the range of 3 j 11 18 hz while cis hydrogens couple in the 3 j 6 15 hz range.
When a set of hydrogens is coupled to two or more sets of nonequivalent neighbors the result is a phenomenon called complex coupling a good illustration is provided by the 1 h nmr. Chemical shift d type of proton examples chemical shift in ppm comments. But vicinal coupling refers to the coupling of two hydrogen atoms that are bound to two adjacent carbon atoms. The 2 bond coupling between hydrogens bound to the same alkene carbon referred to as geminal hydrogens is very fine generally 5 hz or lower.
1h nmr chemical shifts 11 10 9 8 7 6 5 4 3 2 1 0 rh o h r 2ccr h roch 3 ch 3 rch 3 o rh ch 3 ch nh oh rnh 2 o nh 2 rnh 2 roh o oh roh δ ppm type of c hδ ppm description of proton 0 9 alkyl methyl 1 3 alkyl methy lene 1 5 2alkyl methine 1 8 allylic c is next to a pi bond 2 2 3α to carbonyl c is next to c o 2 3 benzylic c is next. The terms geminal and vicinal coupling come under nmr nuclear magnetic resonance and they describe the differences. 0 8 1 5 ppm alkane c h. The key difference between these two structural components is the number of carbon and hydrogen atoms.