Why Is Compressed Air So Cold

This is perhaps why compressed air cylinders feel cold even before use.
Why is compressed air so cold. Anyone who has ever made use of the compressed air can knows that it can get icy cold. In fact it can become so cold that the cans feature frostbite warnings. Some of you might falsely believe that this happens because the gas expands upon coming out of the can and thus cools off. A gas initially at high pressure cools significantly when that pressure is released.
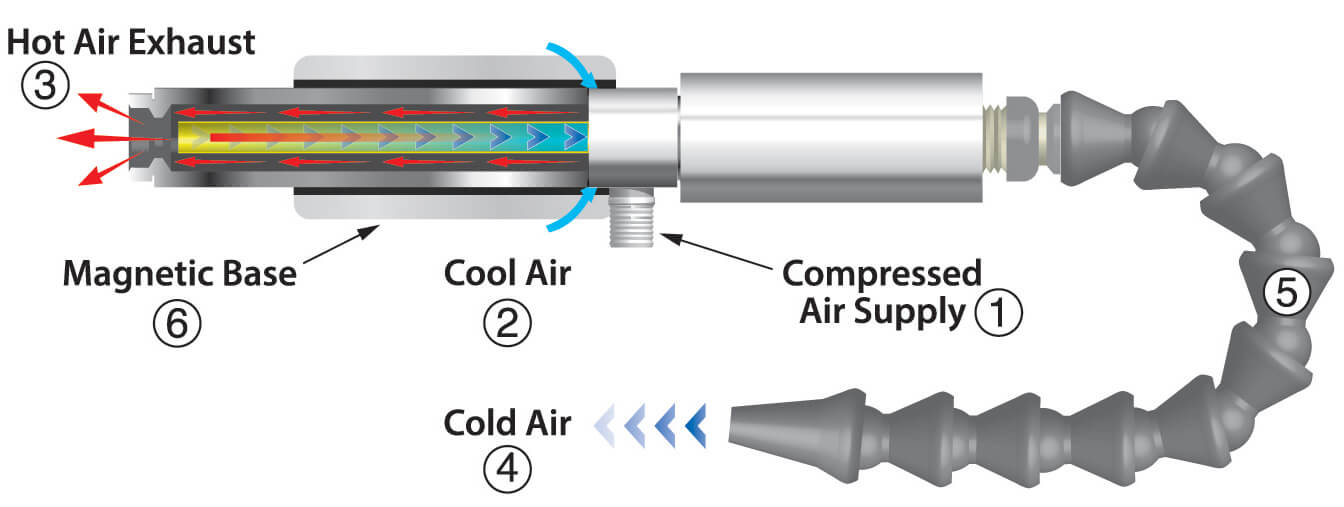
Travelling along this pressure gradient the gas expands and does work and this removes energy from the gas. However that is not true. A fluid such as water or air that rotates around an axis like a tornado is called a vortex. It creates a tornado or vortex of compressed air that separates the fluid into two air streams.
Cans of compressed air get cold while they re discharging because of a thermodynamic principle known as the adiabatic effect. One hot and one cold. Compressed air cylinders are required to be kept out of direct sunlight to avoid gas expansion by direct heating and perhaps also due to phase change although no one seems to mention this explicitly. The cold temperature profile sneaks back towards the can because the air is such a lousy conductor of heat so the heat is all coming from the can.
Minutephysics knows the actual reason why compressed air cans become so cold and will explain it. Eventually your hand gets cold.